2015
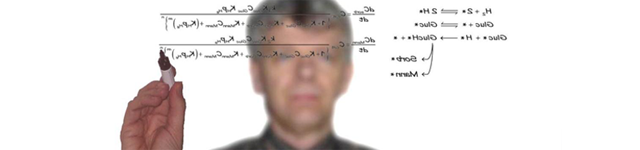
An approach to reconcile the observed reaction kinetics at gas and liquid phase conditions has been investigated for reaction mixtures exhibiting a pronounced thermodynamic non-ideality, i.e., the esterification of acetic acid with methanol. Activity coefficients up to 2.5 were estimated (NRTL) for methanol. The reaction order for methanol in the gas phase was between 0.05 and -0.53, but in the liquid phase 1.0. The kinetic model is based on an Eley-Rideal mechanism. From the kinetic modeling it was found that due to pronounced differences in catalyst occupancies, the enthalpy of the surface species is affected by the transition from G to L phase. This is accounted for by an excess enthalpy estimated by regression.